
Developing combination devices, also called combination products, such as inhalers or injectables, is a complex and interdisciplinary endeavor that lies at the intersection of pharmaceuticals, biologics, engineering, and medical science. These innovative devices are designed to provide patients with a more convenient and effective way to administer medications.
One of the primary objectives in developing combination products is to enhance treatment adherence, particularly in chronic conditions where consistent medication delivery is critical. By combining medication with a delivery system, patients can receive accurate doses, reducing the risk of errors and ensuring that the therapeutic benefits are maximized.
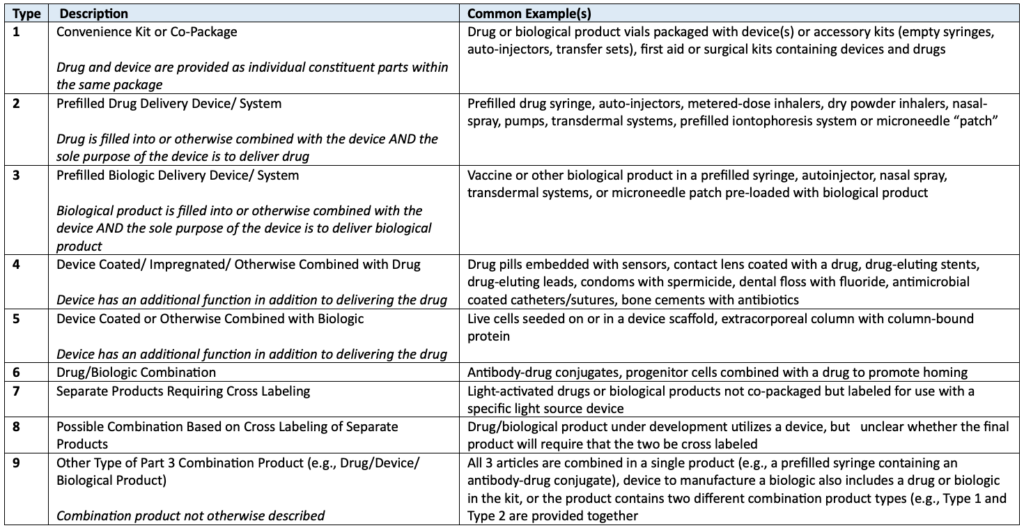
Requirements management tools such as Jama Connect® have become indispensable assets in the product development process, helping streamline the complexities involved in producing safe and effective combination devices.
This article explores how Jama Connect can prove invaluable in areas such as reuse/variant management, hazard library maintenance, compliance standards, integrated risk management, and expeditious reviews, making it an essential tool for engineers and developers in the medical field.
Simplify Complex Traceability
- The development of combination devices often involves integrating a complex hierarchy of requirements, from User Needs and System Requirements, down through Risk Evaluations, Subsystems, and Verifications and Validations. Maintaining proper traceability throughout the product development lifecycle is a vital component of developing safe and effective products. Jama Connect allows development teams to simplify this process by enabling Live Traceability™ between development artifacts. Product development team can establish traceability, ensuring that every design element, from the software components to physical hardware, aligns with the initial requirements. This robust traceability is essential for regulatory compliance and safety, particularly in the medical device industry.
Requirements Reuse
- Combination product teams often face added levels of complexity in their development process as they work to adapt product designs to the specific requirements of multiple applications, and the diverse regulatory demands across markets. Jama Connect allows for the efficient reuse of requirements, hazards, risk assessments, and verification testing across projects, enabling development teams to take a platform approach to their development process, track the evolution of variants, assess the impact of change across all their systems, and ensure compliance with international standards is maintained. This significantly reduces redundancy, minimizes errors, and speeds up development cycles.
Compliance with Standards
- Combination devices must adhere to stringent regulatory and quality standards, such as ISO 13485, ISO 14971, ISO 11608, and FDA requirements. Jama Connect aids in aligning the project with these standards by providing tools for document control, validation, and verification. It supports the creation of audit trails, which are essential for proving compliance. This streamlines the certification process and minimizes the risk of non-compliance.
Integrated Risk Management
- Risk Management is a critical component of developing combination products, but development teams often struggle with highly disconnected risk management processes. Jama Connect’s integrated risk management capabilities allow teams to proactively identify, assess, and mitigate risks throughout the development process, and ensure Live Traceability between risk evaluations and controls. Risk matrices, failure mode and effects analysis (FMEA), and other risk management methodologies can be seamlessly integrated into the development workflow, ensuring that potential issues are addressed early and efficiently.
Hazard Library Management
- Safety is of paramount importance in the development of combination products. Maintaining a comprehensive library of hazards, both known and potential, is crucial to mitigate risks effectively. Jama Connect facilitates the organization and accessibility of this critical information. It allows engineers to define, document, and classify hazards and their corresponding risk assessments. This central repository ensures that hazard management remains an integral part of the design process and that safety remains a top priority.
Efficient Reviews
- Efficient and timely reviews are vital in the development of combination devices, as they help uncover issues, assess design tradeoffs, and ensure alignment with requirements. Jama Connect simplifies the review process by offering a collaborative platform for stakeholders to provide feedback, track changes, and sign off on design decisions. This collaborative approach fosters effective communication and reduces the time required for reviews, expediting the overall development cycle.
Developing combination products, which encompass a wide range of medical innovations, requires a multidisciplinary approach and careful management of various elements. Jama Connect is a powerful requirements management tool that streamlines the development process in multiple ways, from facilitating requirements reuse and variant management to managing hazard libraries, ensuring compliance, and integrating risk management.
By leveraging Jama Connect, product development teams can significantly improve their efficiency, reduce errors, enhance safety, and expedite the development of combination products while maintaining the highest standards of quality and regulatory compliance.






Add comment